Medical device manufacturers face an increasing amount of regulatory barriers to market their products. One of those is the requirement to identify an Authorised Representative (REP) in the EU, Switzerland, and UK. While in the past it was sufficient to have one EC-REP for the whole of Europe, due to the Brexit and non-extension of the MRA between Switzerland and the EU, today manufacturers need an EC-REP, CH-REP, and UK-REP if they are not located within one of the jurisdictions. That’s three times the amount before! That is not only an administrative nightmare – it also takes up valuable space on the already crowded products label.
But at last, there is one good news. Due to the special legal situation of Liechtenstein a company from Liechtenstein can act as EC-REP and CH-REP at the same time!
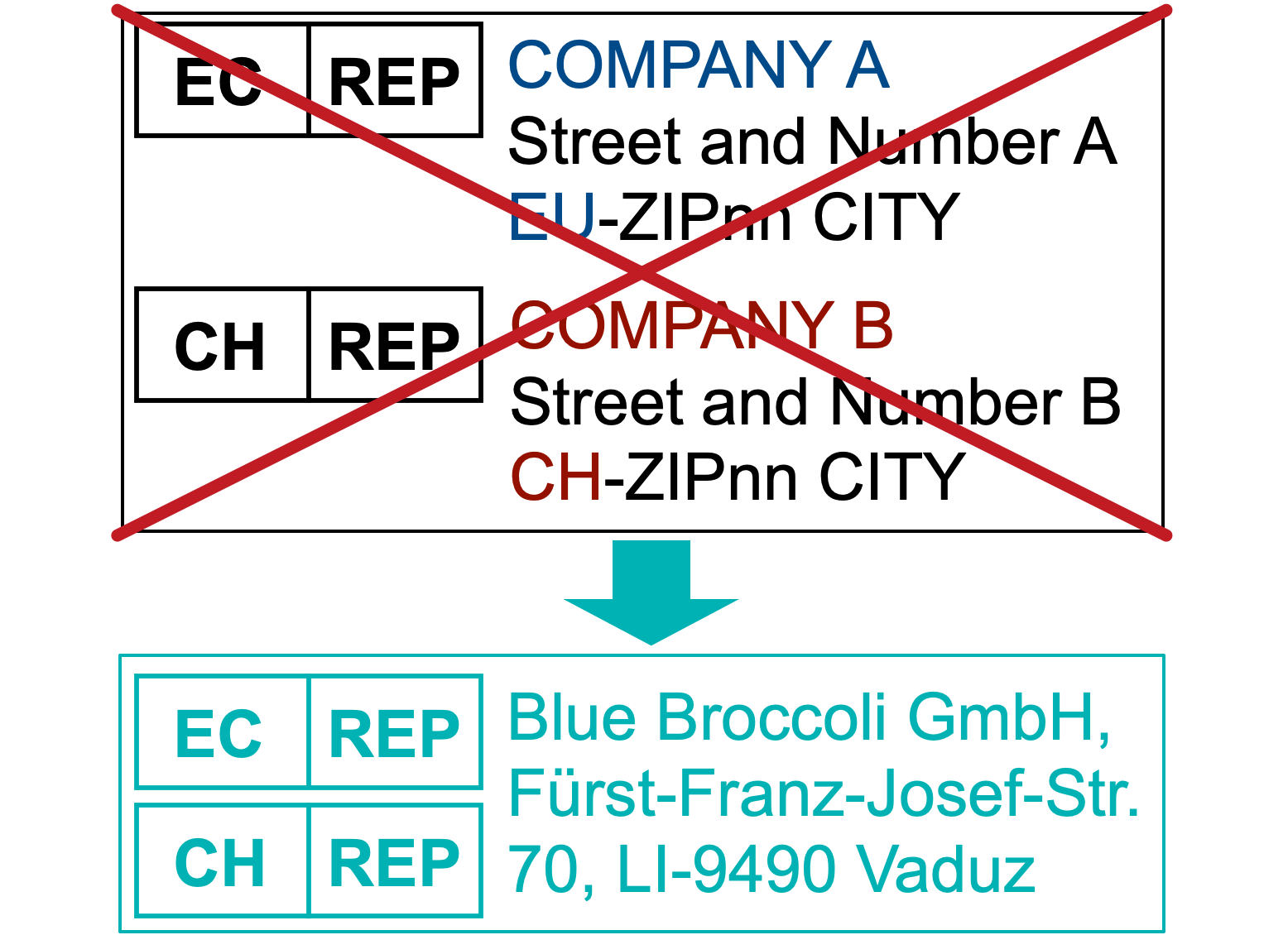
How is that possible?
Liechtenstein is a member of the European Economic Area and as such fully implemented the EU MDR and IVDR and is seen as a member state under the MDR and IVDR. Therefore a company from Liechtenstein can act as a manufacturer, importer, authorised representative, and distributor in the EU and EEA. Blue Broccoli was for example the first company that registered in the EUDAMED as an EU Authorised Representative (EC Rep) as written in our blog entry.
At the same time Liechtenstein has a an agreement with Switzerland „Zollvertrag zwischen der Schweiz und dem Fürstentum Liechtenstein“ which basically builds a custom union between Liechtenstein and Switzerland. Annex I of this agreement (published March 24, 2022 in www.gesetze.li > LR-Nr 170.551.631) refers to the Swiss SR 812.213 Medizinprodukteverordnung (MepV – English: Medical Devices Ordinance (MedDO)) as applicable Swiss Law in Liechtenstein. Therefore a company from Liechtenstein can act as a manufacturer, importer, authorised representative, and distributor in Switzerland.
This special status has been confirmed by the EU and by the competent authorities of Liechtenstein and Switzerland.*
Use our unique EC-REP, CH-REP, and UK Rep Service
Do you want to reduce administrative work or safe space on your product label by using a single address for EC-REP and CH-REP?
Get a quick and non-binding quote through the link below!
Importer and other business opportunities
Are you interested to explore further business opportunities through this special legal situation in Liechtenstein? Contact us for business inquiries related to medical devices.

