Our latest Stories and News
Does class I medical device software still exist under the MDR?
EU guidance documents only mention one example of a class I medical device software. A conception support software (MDCG 2019-11). Are there more? In short: Yes, but… Roughly summarised the MDR defines medical devices as devices that are intended for diagnosis or...
MHRA is delaying the UK MDR 2002 for one year
MHRA is delaying the UK MDR 2002 for one year. That means CE marked MedicalDevices (under MDD/IVD/AIMDD/MDR/IVDR) can still be sold in the UK under the CE mark until July 1 2024 - instead of 2023. After that (still not finally confirmed) date the UKCA (under the UK...
Can an AI do your marketing? A 45 min Self-Test
Update May 6, 2024: It’s fascinating how rapidly AI technologies evolve! Not even two years have passed, and already the landscape of AI text-to-image generators has transformed. The interfaces are now more accessible, allowing users to create stunning visual content...
The story of our Name – BLUE BROCCOLI
What do clouds, colour swaps, and domain endings have to do with our company name? Find out the story behind Blue Broccoli! Three main elements formed our name. There is no priority on how much each of the elements contributed to the name and which came first. ...
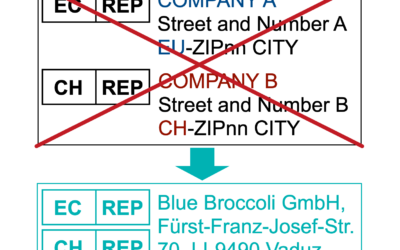
EC-REP and CH-REP with ONE ADDRESS
Medical device manufacturers face an increasing amount of regulatory barriers to market their products. One of those is the requirement to identify an Authorised Representative (REP) in the EU, Switzerland, and UK. While in the past it was sufficient to have one...
SCHWEIZER MEDTECH AUF «DRITTSTAAT» ZURÜCKGESTUFT
Schweizer Medizinprodukte Export in Gefahr! Hersteller müssen ab dem 26. Mai 2021 einen EU Bevollmächtigten auf den Produkten ausweissen um in der EU zu verkaufen!!! Blue Broccoli GmbH bietet diesen Service grenznah und bequem aus Liechtenstein an. Kontaktieren Sie...